Engineering fluorescent reporters for low-oxygen imaging
Motivation: The great oxygenation event spawned the emergence of O2-dependent life on earth as we know it. However, several fascinating and physiologically relevant biosystems continues to thrive in O2-free (anaerobic) or low-O2 (hypoxic) conditions. The poor aqueous solubility of O2 (7.6 ppm in air-saturated water) coupled with its high reactivity and restricted diffusion in (poorly vascularized) biological tissues can lead to the rapid emergence of anaerobic zones. For instance, large tracts of the mammalian gut are almost entirely anaerobic and have supported the emergence of a complex microbial ecology that profoundly impacts health and disease. Several pathogens exploit hypoxia, frequently associated with soft tissue infections to actively combat the action of antibiotics. Despite the physiological significance of hypoxia, our ability to study cells in low-to-no O2 conditions has been hindered by a lack of reliable probes that can facilitate biomolecular imaging in these conditions. Application of widely used genetic reporters such as the green fluorescent protein (GFP) in the context of low-O2 biosystems is constrained by a fundamental challenge – GFP is chemically dependent on molecular O2 for light production. For these reasons, anaerobic biosystems have remained largely “invisible” to the prevalent biomolecular imaging toolset.
Our approach: As a first step towards addressing the above challenge, we and others have engineered a unique class of photosensory proteins to fluoresce without a need for molecular oxygen (Fig. 1). These photoreceptors are derived from light, oxygen, and voltage (LOV) sensing proteins, and they acquire fluorescence simply by binding flavin (vitamin B2) molecules endogenously available in most cells. In addition to engineering bright & photostable LOV reporters (Fig. 2), we have shown that LOV proteins are characterized by several noteworthy advantages including high pH tolerance and thermal stability, rapid maturation kinetics, and a small, compact structure (Fig. 3).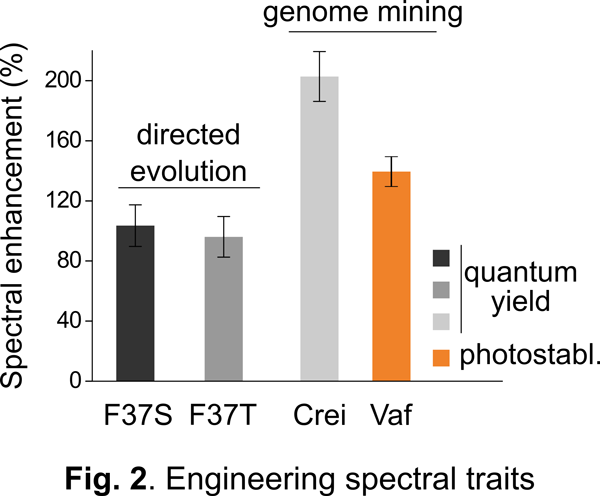 Despite their promise, the current LOV toolbox remains inadequate in terms of several key photophysical & biochemical properties, spectral range, and molecular versatility. To address this challenge, my laboratory pursues a variety of approaches integrating molecular & metabolic engineering, kinetic modeling, directed evolution, and synthetic biology to engineer the next generation of robust LOV reporters and biomolecular sensors for anaerobic imaging.
Despite their promise, the current LOV toolbox remains inadequate in terms of several key photophysical & biochemical properties, spectral range, and molecular versatility. To address this challenge, my laboratory pursues a variety of approaches integrating molecular & metabolic engineering, kinetic modeling, directed evolution, and synthetic biology to engineer the next generation of robust LOV reporters and biomolecular sensors for anaerobic imaging.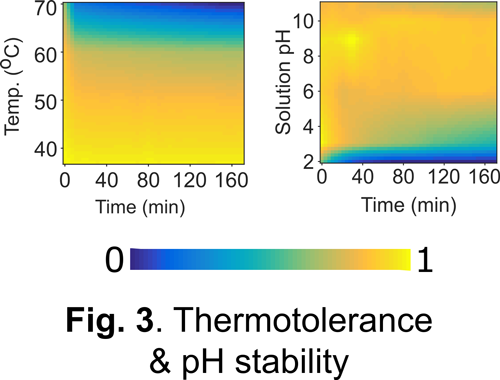 In addition, we actively collaborate with research groups on and off-campus to apply the next generation of anaerobic reporters developed in our lab to study signaling, metabolism, and cell-cell communication in low-O2 environments, with a specific focus on antibiotic resistance and the gut microbiome.
In addition, we actively collaborate with research groups on and off-campus to apply the next generation of anaerobic reporters developed in our lab to study signaling, metabolism, and cell-cell communication in low-O2 environments, with a specific focus on antibiotic resistance and the gut microbiome.